| |
Micro-arc Oxidation / Plasma Electrolytic Oxidation System
 
 
 
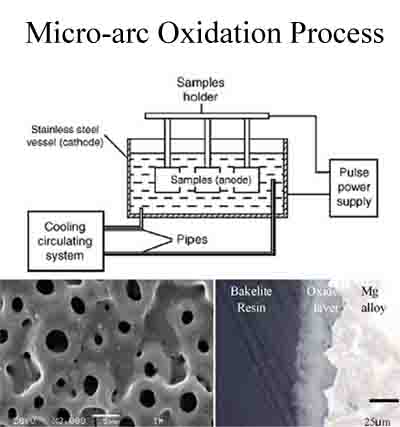
Micro-arc oxidation (MAO) or Plasma Electrolytic Oxidation (PEO) is a plasma-chemical and electrochemical process. The process combines electrochemical oxidation with a high voltage spark treatment in an alkaline electrolyte, resulting in the formation of a physically protectiveoxide film on the metal surface to enhance wear and corrosion resistance as well as prolonging component lifetime. It is especially suitable for the surface oxidation and pigmentation of aluminum, titanium, niobium, zirconium, magnesium and their alloys. The treated components are used in buildings, mechanics, transportations and energy sectors. The technology is simple and energy saving and offers high throughput, low cost, high film quality, wide range of color pigmentation as well as environmental friendliness.
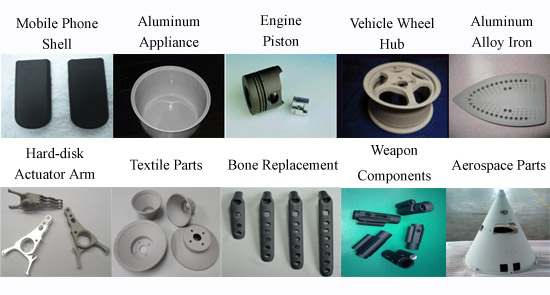
Applications:
Widely used to enhance the wear, thermal, and corrosion resistance as well as
visual appearance of industrial components used in the defense, aerospace,
textile, mechanics, transportation, building, chemical, and biomedical industry.
|
MAO Colors |
 |
Main specifications:
|
Oxidation bath: |
can be designed and constructed according to the processes |
|
Power supply: |
biopolar pulse mode, Solid-state IGBT switching |
|
Positive polar: |
100 - 800 V |
|
Negative polar: |
20 - 200 V |
|
Mode of output: |
constant current, constant voltage and constant power |
| Frequency: |
30Hz - 2kHz |
| (+)/(-) ratio: |
1:1 - 15:1 |
| Duty cycle: |
10% - 80% |
| Output power: |
20kW, 30kW, 50kW, 100kW, 200kW, 400kW |
| Input power: |
3 phases, 380V 50/60Hz |
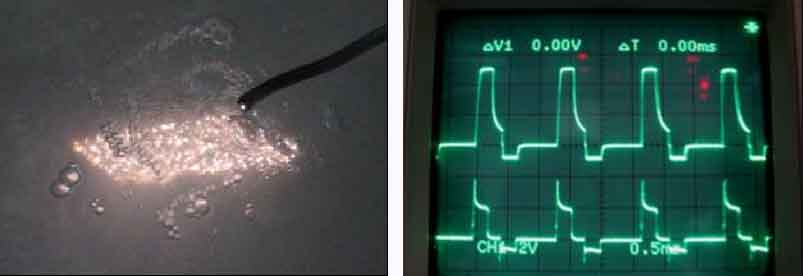
Micro-arc Oxidation MAO treatment Video
Practical Reference:
Degrease - Rinse - Oxidation - Rinse - Dry up
| Terms |
|
Parameters |
Remarks |
| Applicable Materials |
: |
Al, Ti, Mg and their alloys |
/ |
| Electrolyte Composition |
: |
Main Salt with additive |
Main Salt: Sodium Silicate or Sodium Phosphate; Different additives can result in different colors and properties |
| Electrolyte Concentration |
: |
5 - 50 g/litre for main salt |
The higher the concentration, the faster the oxidation rate and lower rise-up temperature |
| Electrolyte pH value |
: |
8 - 13, Alkaline |
/ |
| Working Voltage |
: |
200 V - 700 V |
Different alloy and recipe can use different discharge voltage; The surface morphology and oxide properties can be different under different voltage |
| Current Density |
: |
0.1 - 15 A/dm2 |
/ |
| Oxidation Rate |
: |
30 -150 um/h |
/ |
| Power Consumption |
: |
0.008 - 0.1 kWh/um.dm2 |
/ |
| Temperature |
: |
< 50 degree C |
Cooling system is needed as large amount of heat will be released during the oxidation process |
| Oxidation Time |
: |
10 - 60 minutes |
Longer time with denser layer but with rougher surface |
| Thickness |
: |
10 um - 200 um |
/ |
| Reaction Tank |
: |
Reinforced Polypropylene |
/ |
| Anode Fixator |
: |
Aluminium alloy |
/ |
| Cathode Materials |
: |
Stainless steel |
/ |
| Process Procedures |
: |
Degrease - Rinse - Oxidation - Rinse - Shielding - Dry up |
/ |
| Hardness |
: |
500 HV - 3000 HV |
/ |
| Electrical Isolation |
: |
> 100 MegaOhm |
/ |
| Corrosion Resistance |
: |
> 500h salt fog test |
/ |
| Microstructure |
: |
Crystalline |
/ |
| Surface Status |
: |
Rough surface sample can become relatively smooth while smooth surface will become relatively rough after MAO treatment |
/ |
Related Scientific Articles:
-
F. Y. Jin, H. H. Tong, L. R. Shen, K. Wang, and P. K. Chu, "Micro-structural and Dielectric Properties of Porous TiO2 Films Synthesized on Titanium Alloys by Micro-Arc Discharge Oxidization", Materials Chemistry and Physics, vol. 100, no. 1, pp. 31 - 33 (2006).
-
F. Y. Jin, P. K. Chu, G. D. Xu, J. Zhao, D. L. Tang, H. H. Tong, "Structure and Mechanical Properties of Magnesium Alloy Treated by Micro-Arc Discharge Oxidation Using Direct-Current and High-Frequency Bipolar Pulsing Modes", Materials Science and Engineering A, vol. 435 - 436, no. 1 - 2, pp. 123 - 126 (2006).
-
F. Y. Jin, P. K. Chu, H. H. Tong, and J. Zhao, "Improvement of Surface Porosity and Properties of Alumina Films by Incorporation of Fe Micrograins in Micro-Arc Oxidation", Applied Surface Science, vol. 253, no. 2, pp. 863 - 868 (2006).
-
C. B. Wei, X. B. Tian, S. Q. Yang, X. B. Wang, R. K. Y. Fu, and P. K. Chu, "Anode Current Effects in Plasma Electrolytic Oxidation", Surface and Coatings Technology, vol. 201, no. 9 - 11, pp. 5021 - 5024 (2007).
-
T. Qiu, X. L. Wu, F. Y. Jin, A. P. Huang, and P. K. Chu, "Self-Assembled Growth of MgO Nanosheet Arrays via a Micro-Arc Oxidation Technique", Applied Surface Science, vol. 253, no. 8, pp. 3987 - 3990 (2007).

For additional product information and pricing contact our specialists at sales@plasmatechnol.com.
|

